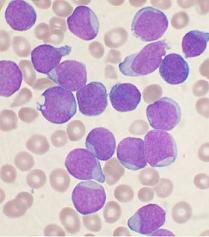
 Blinatumomab is a monoclonal antibody produced by the pharmaceutical company Micromet, which is based in Rockville, Maryland and Munich, Germany. Blinatumomab is designed to enhance the function of the body’s immune system against cancer cells. Specifically, it stimulates cytotoxic T-lymphcytes to target the CD19 antigen, which is a protein expressed on precursor B-cell acute lymphoblastic leukemia cells. Acute lymphoblastic leukemia (ALL) is a rare but deadly cancer that affects the blood and bone marrow of patients and causes anemia, bleeding, and a predisposition to infection. On a yearly basis, there is about 5,760 people in the United States that have adult ALL leukemia. Toxic chemotherapy has been the standard treatment for the disease. Remission is very rare for this deadly malignancy. In patients that have relapsed adult ALL leukemia, there have been very few advances in treatment within the past 30 years. The average five-year survival rate for relapsed adult ALL leukemia is less than 7%. Results of a phase 2 clinical trial, to be presented today June 11, 2011 at the 16th annual meeting of the European Hematology Association in London, shows that blinatumomab can produced a complete remission in relapsed adult ALL leukemia at a rate of 75%. Standard chemotherapy produces a complete remission in relapsed ALL leukemia at a rate of 17 to 45%. Dr. Max Topp, from the University of Wuerzburg in Germany, is the lead investigator for the clinical trial. The study is designed to investigate the efficacy, safety, and tolerability of blinatumomab in adult patients with precursor B cell ALL who relapsed after at least induction and consolidation treatment or who have refractory disease. In the study, patients received blinatumomab for 28 days followed by two weeks without treatment. Patients who had a complete remission after two cycles of treatment went on to receive consolidation with either three additional cycles of blinatomomab or allogeneic stem cell transplantation. The investigational phase 2 clinical trial is still enrolling participants. Currently, there have been 12 participants in the study with 9 participants achieving a complete molecular response with no evidence of leukemic cells in their peripheral blood or in their bone marrow. Dr. Topp has stated, “Relapsed/refractory acute lymphoblastic leukemia is a difficult to treat disease that has seen no meaningful improvement in decades. He also added “To date blinatumomab has shown an impressive and unprecedented level of efficacy in a patient population with limited therapeutic options”. These results are promising and encouraging, but one critique of the study would be the very small number of participants that have been enrolled so far. Only 12 patients have participated, which is too small of a number to make these results statistically significant. Although, the results are promising and hopefully this ongoing investigation will continue to yield treatment successes. Micromet has received approval from the European Medicines Agency to investigate blinatumomab for the treatment of acute lymphoblastic leukemia, mantle cell lymphoma and chronic lymphatic leukemia and from the U.S. Food and Drug Administration for the treatment of acute lymphoblastic leukemia, chronic lymphocytic leukemia and indolent B cell lymphoma. The stock price of Micromet rose by 13% on the Nasdaq after the press release.
Blinatumomab is a monoclonal antibody produced by the pharmaceutical company Micromet, which is based in Rockville, Maryland and Munich, Germany. Blinatumomab is designed to enhance the function of the body’s immune system against cancer cells. Specifically, it stimulates cytotoxic T-lymphcytes to target the CD19 antigen, which is a protein expressed on precursor B-cell acute lymphoblastic leukemia cells. Acute lymphoblastic leukemia (ALL) is a rare but deadly cancer that affects the blood and bone marrow of patients and causes anemia, bleeding, and a predisposition to infection. On a yearly basis, there is about 5,760 people in the United States that have adult ALL leukemia. Toxic chemotherapy has been the standard treatment for the disease. Remission is very rare for this deadly malignancy. In patients that have relapsed adult ALL leukemia, there have been very few advances in treatment within the past 30 years. The average five-year survival rate for relapsed adult ALL leukemia is less than 7%. Results of a phase 2 clinical trial, to be presented today June 11, 2011 at the 16th annual meeting of the European Hematology Association in London, shows that blinatumomab can produced a complete remission in relapsed adult ALL leukemia at a rate of 75%. Standard chemotherapy produces a complete remission in relapsed ALL leukemia at a rate of 17 to 45%. Dr. Max Topp, from the University of Wuerzburg in Germany, is the lead investigator for the clinical trial. The study is designed to investigate the efficacy, safety, and tolerability of blinatumomab in adult patients with precursor B cell ALL who relapsed after at least induction and consolidation treatment or who have refractory disease. In the study, patients received blinatumomab for 28 days followed by two weeks without treatment. Patients who had a complete remission after two cycles of treatment went on to receive consolidation with either three additional cycles of blinatomomab or allogeneic stem cell transplantation. The investigational phase 2 clinical trial is still enrolling participants. Currently, there have been 12 participants in the study with 9 participants achieving a complete molecular response with no evidence of leukemic cells in their peripheral blood or in their bone marrow. Dr. Topp has stated, “Relapsed/refractory acute lymphoblastic leukemia is a difficult to treat disease that has seen no meaningful improvement in decades. He also added “To date blinatumomab has shown an impressive and unprecedented level of efficacy in a patient population with limited therapeutic options”. These results are promising and encouraging, but one critique of the study would be the very small number of participants that have been enrolled so far. Only 12 patients have participated, which is too small of a number to make these results statistically significant. Although, the results are promising and hopefully this ongoing investigation will continue to yield treatment successes. Micromet has received approval from the European Medicines Agency to investigate blinatumomab for the treatment of acute lymphoblastic leukemia, mantle cell lymphoma and chronic lymphatic leukemia and from the U.S. Food and Drug Administration for the treatment of acute lymphoblastic leukemia, chronic lymphocytic leukemia and indolent B cell lymphoma. The stock price of Micromet rose by 13% on the Nasdaq after the press release.







 DrSamGirgis.com is a blog about medicine, nutrition, health, wellness, and breaking medical news. At DrSamGirgis.com, the goal is to provide a forum for discussion on health and wellness topics and to provide the latest medical research findings and breaking medical news commentary.
DrSamGirgis.com is a blog about medicine, nutrition, health, wellness, and breaking medical news. At DrSamGirgis.com, the goal is to provide a forum for discussion on health and wellness topics and to provide the latest medical research findings and breaking medical news commentary.
{ 0 comments… add one now }