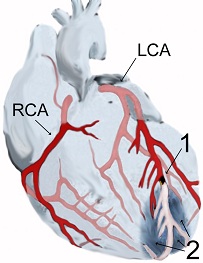
 Coronary artery disease (CAD) is the single leading cause of death in the United States and in many countries throughout the world. There are over 1.2 million heart attacks in the United States every year, and about 34% of individuals that have a heart attack will die of it according to the American Heart Association. CAD develops as a result of narrowing, or stenosis of the arteries that supply blood to the heart. This process is known as atherosclerosis. As the coronary arteries become stenotic, they are at risk of becoming completely occluded which will result in a heart attack, or myocardial infarction. The heart cells that are damaged usually die and scar tissue takes their place in the heart. Damage as a result of a heart attack is usually permanent, and weakens the heart so that the heart doesn’t pump as well, a condition known as congestive heart failure. We have previously discussed experiments looking at the use of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) to stimulate collateral blood vessels growth in the heart to treat CAD. In recent research, scientists from University College London and Harvard Medical School lead by Dr. Paul Riley showed that cardiac stem cells can be induced to repair damage after a heart attack. The results were published today, June 8, 2011 online in the journal Nature. Heart progenitor stem cells, known as epicardium-derived progenitor cells (EPDCs), are found in the lining of the heart and are usually dormant. The researchers used a naturally occurring protein called thymosin beta 4 (Tβ4) to bring the EPDCs out of dormancy and induce them to become mature heart cells (cardiomyocytes). In the experiments, the scientists infused Tβ4 into mice for several days and then caused heart attacks by blocking arteries that supply the heart with blood. In the mice that were infused with the Tβ4, the heart progenitor stem cells were activated and migrated from the epicardium to the area of damage and became mature cardiomyocytes. In the mice that did not receive Tβ4, this did not occur. In addition, the scientist were able to show that the mice treated with Tβ4 had less heart damage as a result of the heart attack and had 25% greater heart function, known as ejection fraction. Thymosin beta 4 is already in clinical trial for the treatment of coronary artery disease at the time of a heart attack. Dr. Riley envisions that Tβ4 can be used in high risk patients prior to developing a heart attack as a preventative medication, similar to aspirin and cholesterol medications. The exact mechanism whereby Tβ4 induces epicardium-derived progenitor cells to become active, migrate, and mature into cardiomyocytes is unclear at this point. The mechanism is proposed to occur by an epigenetic effect that causes a change to DNA and alters gene expression. There is currently a search for other proteins and medications which can perform the same function as Tβ4 but with more potency and efficiency. The authors of the research paper wrote, “The identification of bona fide source of myocardial progenitors is a significant step towards resident-cell-based therapy for acute myocardial infarction in human patients”.
Coronary artery disease (CAD) is the single leading cause of death in the United States and in many countries throughout the world. There are over 1.2 million heart attacks in the United States every year, and about 34% of individuals that have a heart attack will die of it according to the American Heart Association. CAD develops as a result of narrowing, or stenosis of the arteries that supply blood to the heart. This process is known as atherosclerosis. As the coronary arteries become stenotic, they are at risk of becoming completely occluded which will result in a heart attack, or myocardial infarction. The heart cells that are damaged usually die and scar tissue takes their place in the heart. Damage as a result of a heart attack is usually permanent, and weakens the heart so that the heart doesn’t pump as well, a condition known as congestive heart failure. We have previously discussed experiments looking at the use of vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) to stimulate collateral blood vessels growth in the heart to treat CAD. In recent research, scientists from University College London and Harvard Medical School lead by Dr. Paul Riley showed that cardiac stem cells can be induced to repair damage after a heart attack. The results were published today, June 8, 2011 online in the journal Nature. Heart progenitor stem cells, known as epicardium-derived progenitor cells (EPDCs), are found in the lining of the heart and are usually dormant. The researchers used a naturally occurring protein called thymosin beta 4 (Tβ4) to bring the EPDCs out of dormancy and induce them to become mature heart cells (cardiomyocytes). In the experiments, the scientists infused Tβ4 into mice for several days and then caused heart attacks by blocking arteries that supply the heart with blood. In the mice that were infused with the Tβ4, the heart progenitor stem cells were activated and migrated from the epicardium to the area of damage and became mature cardiomyocytes. In the mice that did not receive Tβ4, this did not occur. In addition, the scientist were able to show that the mice treated with Tβ4 had less heart damage as a result of the heart attack and had 25% greater heart function, known as ejection fraction. Thymosin beta 4 is already in clinical trial for the treatment of coronary artery disease at the time of a heart attack. Dr. Riley envisions that Tβ4 can be used in high risk patients prior to developing a heart attack as a preventative medication, similar to aspirin and cholesterol medications. The exact mechanism whereby Tβ4 induces epicardium-derived progenitor cells to become active, migrate, and mature into cardiomyocytes is unclear at this point. The mechanism is proposed to occur by an epigenetic effect that causes a change to DNA and alters gene expression. There is currently a search for other proteins and medications which can perform the same function as Tβ4 but with more potency and efficiency. The authors of the research paper wrote, “The identification of bona fide source of myocardial progenitors is a significant step towards resident-cell-based therapy for acute myocardial infarction in human patients”.
See the YouTube video released by the British Heart Foundation which documents the results:
Reference:
Nicola Smart et. al. “De novo cardiomyocytes from within the activated adult heart after injury” Nature online June 8, 2011 doi:10.1038/Nature10188







 DrSamGirgis.com is a blog about medicine, nutrition, health, wellness, and breaking medical news. At DrSamGirgis.com, the goal is to provide a forum for discussion on health and wellness topics and to provide the latest medical research findings and breaking medical news commentary.
DrSamGirgis.com is a blog about medicine, nutrition, health, wellness, and breaking medical news. At DrSamGirgis.com, the goal is to provide a forum for discussion on health and wellness topics and to provide the latest medical research findings and breaking medical news commentary.
{ 1 comment… read it below or add one }
Amazing!!